News flash. Two years late. Diamond is not the hardest known material. There are at least three known substances that are harder: Rhenium Diboride, Ultrahard Fullerite and Aggregated Diamond Nanorods.
I’m a little worried. I think this is what happens when you grow older. Technology has just outdated one of those simple scientific truths I learned about in school. What’s worse is that it took me almost two years to find out about it.
But before I get into a self-pitying “science is for the young” groove, let me tell you what I’ve learned so far.
First, a big thank you to Business Week. Yes, that’s right, Business Week. Not known for it’s scientific coverage, but the May 7, 2007 issue had a snippet on page 79 about the successful effort to create a substitute for industrial diamonds for slicing through steel. Apparently, the diamond reacts with the steel to form by-products that dull the blade. Scientists at UCLA have discovered a mixture of Boron and Rhenium that is hard enough to scratch diamond, and doesn’t react with steel. Press release dates to April 19, 2007, so it’s a pretty recent discovery.

In all fairness, Rhenium DiBoride is only harder than diamond in certain directions, due to its layered structure. But reading about it sent me to the web – what other substances have been discovered that are harder than diamond? Somehow, learning that diamond wasn’t the hardest material bar none made me realize that I last took Material Science coursework at Stanford in 1992.
Fortunately, in the 15 years since that coursework, a lot has happened to help me get up to speed in a matter of minutes. And I am glad I did, because new materials are just too cool.
First, let’s start with the simpler one: Ultrahard Fullerite. Fullerene is a form of carbon based on the 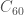 structure of buckyball-fame. From Wikipedia:
structure of buckyball-fame. From Wikipedia:
Ultrahard fullerite ( ) is a form of carbon which has been found to be harder than diamond, and which can be used to create even harder materials, such as aggregated diamond nanorods.
) is a form of carbon which has been found to be harder than diamond, and which can be used to create even harder materials, such as aggregated diamond nanorods.
Specifically, it is a unique version of fullerene (which is a class of spherical, ellipsoidal, or tubular carbon molecules) with three-dimensional polymer bonds. This should not be confused with P-SWNT fullerite, which is also a polymerized version of fullerene. It has been shown[1][2] that when testing diamond hardness with a scanning force microscope of specific construction, ultrahard fullerite can scratch diamond.
Very cool, but now, of course I’m thinking, “Tell me more about these aggregated diamond nanorods!” (I’m sure you were thinking the same thing.)

That, my friends, is a thing of beauty. According to this article at the European Synchotron Radiation Facility, Aggregated Diamond Nanorods are the least-compressible known material. To be specific, the density of ADNR is 0.2% to 0.4% greater than Diamond. ADNR is also 11% less compressible than diamond, and has an isothermal bulk modulus of 491 GPa (gigapascals) compared to just 442 for diamond.
Of course, I’m only reading about this now. PhysicsWeb.org had the coverage on this discovery in Germany back on August 26, 2005. (it’s actually a very clear & well written piece.) You can bet that the PhysicsWeb RSS feed is going into my reader tonight…
Wikipedia has a very nice summary here as well.
Oh well, better late than never. My guess is that one or two people out there also missed this, which is why I’m posting it tonight.
Now, I think we just need to find a way to start a luxury jewelry business that specializes in ADNR-based engagement rings. Why settle for diamond, which can get scratched so easily? We could make a fortune on this one on the high end…
Update (1/4/2010): See the comment from January 2010 below, but it seems Rhenium DiBoride is no longer assessed as harder than diamond.


